Sixth-form student Harry shares his insights following a week of work experience at Scitech.
Hi, I’m Harry, a Year 12 student currently studying A-levels in Geography, Art and Maths with aspirations of a potential career in architecture in the future. Thanks to a work experience programme arranged by my school and Scitech, I was fortunate enough to spend a week in the Scitech office. I had the opportunity to work amongst many professionals and the chance to learn and develop from their advice and input.
What did I do?
My experience was mainly focused on architecture; however, I had an introduction to many different sectors and disciplines, including project management, electrical engineering, and process engineering. I had meetings with a specialist in each area where they gave an insight into their role and responsibilities. These were always very interesting and gave me an insight into the roles that work alongside architects.
As I mentioned before, the bulk of my week was focused on architecture, and this consisted mainly of two parts. First, I was introduced to the architecture sector through a meeting and presentation given by one of the architects at Scitech. This was then followed by my assigned project, which I worked on through most of the week between my meetings with the other disciplines.
My project work
My project helped me to learn and develop many skills. I was assigned a brief created by one of the Scitech architects, with a fictional client and an area of land to model my project on. I had to research and design a production facility for a company by the name of “Happy Pharmaceuticals” for producing OSD (oral solid dose) tablets. I was also given a more detailed day-by-day checklist which helped me to manage my time on the most important things and helped as a starting point for my research.
I started by researching the location of the land and local restrictions on the construction. I then researched the hygiene standards for the process and the production process, including necessary machines, materials and more. Once most of the research had been done, I then progressed to creating designs for the building, starting with rough hand-drawn sketches before moving on to digital floorplans and diagrams showing the flow of materials and people, which can be seen below:
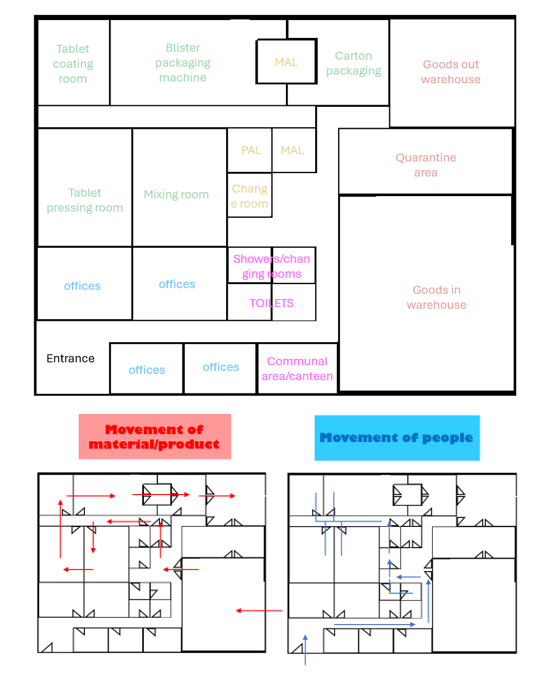
I enjoyed working on my project and was fascinated by my research into the requirements and standards in the production process for pharmaceutical products. I loved being able to transfer my ideas from my head onto the paper and into my designs and then receive constructive and helpful feedback from several architects who assisted me throughout my project. I also received input from several different disciplines throughout the office and developed my ideas with their advice and guidance in mind, which benefitted my project and design greatly.
Presenting my work
At the end of the week, I was very fortunate to be given the opportunity to present my project. I was very intimidated to find out that I would be giving my presentation in front of many people including the Managing Director of the company, however, I was excited as I was confident in my work and wanted to share it with those who attended.
The presentation for me was the most challenging but the most rewarding, part of my whole experience as I had little experience in presenting and was nervous. Despite this, I think I did a good job, and I was very relieved by all the positive feedback I received. I am incredibly grateful for the opportunity to practice my presentation skills in the safe environment provided by Scitech and all who attended. I feel I have left my week there not only with new knowledge and skills but also a new confidence in myself.
Conclusion
My week at Scitech provided numerous valuable insights and experiences, including:
• An insight into the office environment and the cooperation between different sectors and disciplines all working together collectively.
• I was given an important preview of an architectural career and the structure of their tasks, roles and duties, which have been incredibly interesting and crucial for an aspiring architect like myself.
• A beneficial opportunity to develop my speaking and presentation skills which are so important for many forms of employment and education I may choose to pursue in the future.
• A crucial chance to develop time-management and organisational skills. This included project work time, my regularly scheduled discipline insight meetings, as well as the practice of understanding and delivering on a brief within a deadline, which is an incredibly important skill.
I am so grateful to everyone at Scitech for this opportunity, from everyone who helped to organise my visit or shared their knowledge with me. Everyone made me feel welcome in the office and made my visit enjoyable. This experience has exceeded all my expectations and is something I will take with me into the future.
